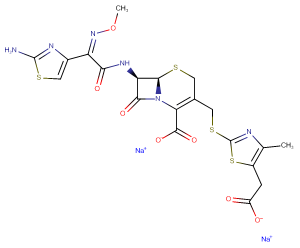
Cefodizime Sodium
CAS No. 86329-79-5
Cefodizime Sodium( —— )
Catalog No. M21331 CAS No. 86329-79-5
Cefodizime Sodium is a third generation cephalosporin antibiotic has broad-spectrum activity.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 45 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCefodizime Sodium
-
NoteResearch use only, not for human use.
-
Brief DescriptionCefodizime Sodium is a third generation cephalosporin antibiotic has broad-spectrum activity.
-
DescriptionCefodizime Sodium is a third generation cephalosporin antibiotic has broad-spectrum activity.(In Vitro):Enterobacteriaceae including Escherichia coli, Klebsiella pneumoniae, Morganella morgan ii, Proteus mirabilis, Proteus vulgaris, Shigella sonnei, Yersinia enterocolitica and Salmonella species are all consistently sensitive to Cefodizime in vitro. Cefodizime has marginal but variable inhibitory activity against Citrobacter species including Citrobacter freundii, and Serratia marcescens. Cefodizime inhibits other Gram-negative bacteria including Haemophilus irifluenzae, Moraxella catarrhalis, Neisseria gonorrhoeae and Neisseria meningitidis.Cefodizime is a bactericidal antibiotic having high affinity for penicillin-binding proteins lA/B, 2 and 3 of E. coli. The in vitro concentrations of Cefodizime resulting in bactericidal activity against susceptible strains of Gram-positive and Gram-negative bacteria are generally similar to the minimum inhibitory concentrations.(In Vivo):In experimentally-induced K. pneumoniae respiratory tract infections in mice, Cefodizime has activity comparable to Cefotaxime and Ceftazidime, and greater than that of Cefoperazone, Latamoxef, Cefuroxime or cefazolin for 8 hours after a single subcutaneous dose of 50 mg/kg. However, unlike these cephalosporins, Cefodizime continues to demonstrate pronounced bactericidal activity for at least 48 hours after a single injection. Complete bacterial clearance from the lung is achieved within 48 hours in 50% of the mice although Cefodizime is no longer detectable in the serum.
-
In VitroEnterobacteriaceae including Escherichia coli, Klebsiella pneumoniae, Morganella morgan ii, Proteus mirabilis, Proteus vulgaris, Shigella sonnei, Yersinia enterocolitica and Salmonella species are all consistently sensitive to Cefodizime in vitro. Cefodizime has marginal but variable inhibitory activity against Citrobacter species including Citrobacter freundii, and Serratia marcescens. Cefodizime inhibits other Gram-negative bacteria including Haemophilus irifluenzae, Moraxella catarrhalis, Neisseria gonorrhoeae and Neisseria meningitidis. Cefodizime is a bactericidal antibiotic having high affinity for penicillin-binding proteins lA/B, 2 and 3 of E. coli. The in vitro concentrations of Cefodizime resulting in bactericidal activity against susceptible strains of Gram-positive and Gram-negative bacteria are generally similar to the minimum inhibitory concentrations.
-
In VivoIn experimentally-induced K. pneumoniae respiratory tract infections in mice, Cefodizime has activity comparable to Cefotaxime and Ceftazidime, and greater than that of Cefoperazone, Latamoxef, Cefuroxime or cefazolin for 8 hours after a single subcutaneous dose of 50 mg/kg. However, unlike these cephalosporins, Cefodizime continues to demonstrate pronounced bactericidal activity for at least 48 hours after a single injection. Complete bacterial clearance from the lung is achieved within 48 hours in 50% of the mice although Cefodizime is no longer detectable in the serum.
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number86329-79-5
-
Formula Weight628.6
-
Molecular FormulaC20H18N6Na2O7S4
-
Purity>98% (HPLC)
-
SolubilityDMSO:1 mg/mL (159.7 mM)
-
SMILESCC1=C(SC(=N1)SCC2=C(N3[C@@H]([C@@H](C3=O)NC(=O)/C(=N\OC)/C4=CSC(=N4)N)SC2)C(=O)[O-])CC(=O)[O-].[Na+].[Na+]
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Henwood J M Monk J P . Enoxacin. A review of its antibacterial activity pharmacokinetic properties and therapeutic use.[J]. drugs 1988 40(3):373-447.
molnova catalog



related products
-
P7C3
The P7C3 class of neuroprotective aminopropyl carbazoles has been shown to block neuronal cell death in models of neurodegeneration.
-
Glycocholic Acid Sod...
Glycocholic Acid Sodium Salt is a conjugated bile salt and ionic biologic detergent. It is involved in the emulsification of fats.
-
PS 48
PS 48 has been shown to be a PKB Kinase (PDK1) activator (Kd: 10.3 μM). This compound selectively binds to the PIF-binding pocket of PKB Kinase (PDK1).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


